A "Driver Switchover" Mechanism of Influenza Virus Transport from Microfilaments to Microtubules.

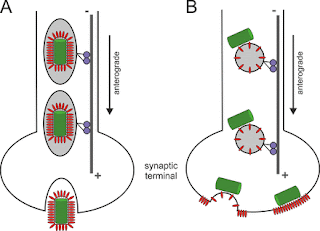
When infecting host cells, influenza virus must move on microfilaments (MFs) at the cell periphery and then move along microtubules (MTs) through the cytosol to reach the perinuclear region for genome release. But how viruses switch from the actin roadway to the microtubule highway remains obscure. To settle this issue, we systematically dissected the role of related motor proteins in the transport of influenza virus between cytoskeletal filaments in situ and in real-time using quantum dot (QD)-based single-virus tracking (SVT) and multicolor imaging. We found that the switch between MF- and MT-based retrograde motor proteins, myosin VI (myoVI) and dynein, was responsible for the seamless transport of viruses from MFs to MTs during their infection. After virus entry by endocytosis, both the two types of motor proteins are attached to virus-carrying vesicles. MyoVI drives the viruses on MFs with dynein on the virus-carrying vesicle hitchhiking. After role exchanges at actin-microtubul